Chapter 2
The chemical context of life
You must know
-
The three subatomic particles and their significance.
-
The types of bonds, how they form, and their relative strengths.
Matter (chất) Vs Energy (năng lượng)
Element (nguyên tố) Vs Compound (hợp chất)
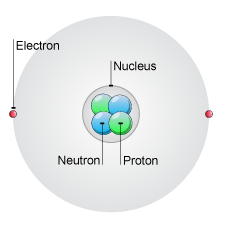
Atomic Structure(cấu trúc nguyên tử)
-
Atom is the smallest unit of matter that retains properties of an element.
Element Notation (kí hiệu nguyên tố)
-
There are two ways the symbol of an element can be represented. Please be familiar with using and understanding both ways.

#1

#2
Isotope (đồng vị)
-
The number of neutrons varies but the number of protons stays the same of the same element. (If the number of protons changes, the properties of the element change as well.)

Chemical Bonds (liên kết)
Strongest Bonds (Intramolecular forces - lực tương tác trong phân tử)
-
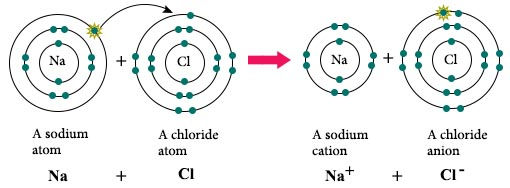
Ionic bonds (liên kết ion)
-
A metal (+) and a non-metal (-) bond.
-
The metal loses electrons to become a positively charged cation (ion dương), whereas the nonmetal accepts those electrons to become a negatively charged anion (ion âm).
-
-
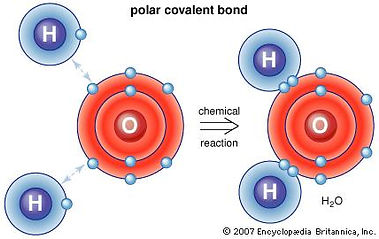
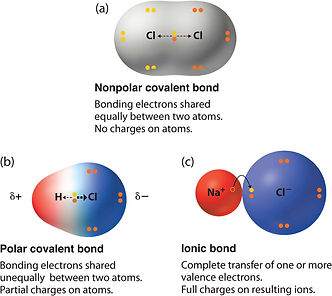
Covalent bonds (liên kết hóa trị)
-
Two non-metals (-) share the electrons.
-
Polar (phân tử phân cực): the covalent bond between the atoms that differ in electronegativity.
-
Nonpolar (phân tử không phân cực): the electrons are equally shared between the atoms.
-
-
Weaker bonds (Intermolecular forces-lực tương tác giữa các phân tử)
-
Hydrogen bonding (liên kết hydro)
-
A hydrogen atom covalently bound to either fluorine, oxygen or nitrogen (F-O-N).
-
Water molecules are bonded to one or another by hydrogen bonding.
-

-
Van der Waals Interactions
-
The weakest intermolecular forces between the molecules.
-
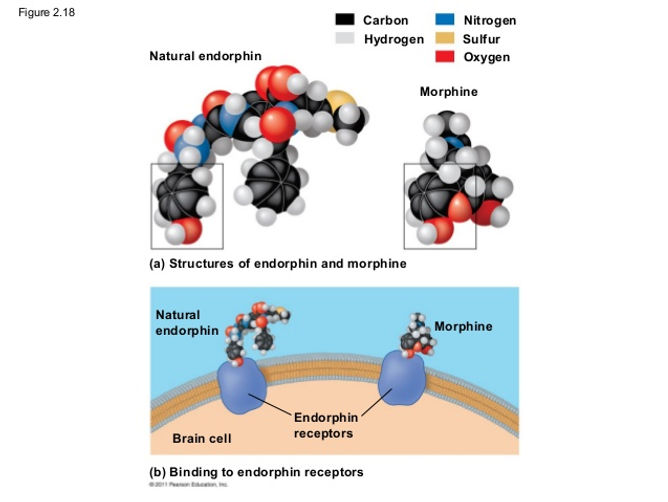
Different bonds give the molecules different structures or shaped. Therefore, they have different functions.
-
Molecules with similar shapes mean that they have similar function.
-
For example: Endorphine and morphine
-
molecules: phân tử
Chemical Reactions (phản ứng hóa học)
Reactants (chất tham gia) -> Products (sản phẩm)
For example:
C6H12O6 + 6O2 -> 6CO2 + 6H2O
There are 5 fundamental types of chemical reactions: synthesis (hóa hợp), decomposition (phân hủy), single and double replacement (thế), acid base (trung hòa), and combustion (tỏa nhiệt).
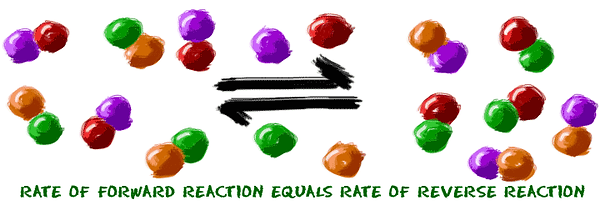
Chemical equilibrium (sự cân bằng)
The rate of the forward reaction is equal to the rate of the backward reaction.
However, the concentration of the reactants isn't the same as the concentration of the products.
Sources:
Reece, J. B., L. A. Urry, M. L. Cain, S. A. Waasserman, P. V. Minorsky, and R. B. Jackson. "The Chemical Context of Life." In Campbell Biology, 28-43. 10th ed. San Francisco, CA: Pearson, 2011.