Chapter 3
Water!!
Polarity of Water (tính phân cực của nước)
-
Water has hydrogen bond (liên kết hydro) because water (H2O) meets the requirements (F-O-N and H).
-
One H2O molecule can bond with another 4 H2O molecules.

Properties of Water
-
There are 6 main properties of water:
-
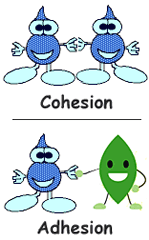
Cohesion and Adhesion:
-
Cohesion is the attraction between two similar molecules
-
For example: water molecule and water molecule .
-
Adhesion is the attraction between two different molecules
-
For example: water molecules and the leaf.
-
-
High specific heat (nhiệt dung riêng):
-
Definition of specific heat:
-
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
-
The specific heat of water is 4.186 joule/gram °C which is higher than any other common substance.
-
This means that it takes more energy to raise the temperature of water by one degree Celsius than for example MgO (0.208 joule/gram °C).
-
The specific heat of water is high is because of hydrogen bonding which is the strongest intermolecular forces.
-
This is important because it helps to moderate temperature.
-
For example: Humans are 65% of water which helps us to stabilise our body temperature.
-
-
-
High heat of vaporization (nhiệt bay hơi):
-
Definition of heat (enthalpy) of vaporization:
-
The quantity of heat that must be absorbed if a certain quantity of liquid is vaporized at a constant temperature.
-
For water to boil to 100 degree Celsius, it requires 2260 J/g.
-
This is important because it helps to moderate temperature.
-
For example: helping plants to cool, humans sweat.
-
-
-
Universal solvent:
-
Because water is polar, it can dissolve nearly all ionic salts and polar molecules.
-
-
Surface tension:
-
It is a measure of tension that is needed to break or stretch the surface of a liquid.
-
This is important because a habitat can survive on the surface of water.
-
-
Low density of Ice:
-
When water becomes solid (ice), it becomes less dense and so it floats!
-
This is important for fishes because this prevents lakes or oceans getting cold.
-
Water gets more dense until 4 degree Celcius but after that it gets less dense again.
-
-


Very important!
Hydrophobic (ghét nước) vs Hydrophilic (yêu nước)
"Like dissolves like"


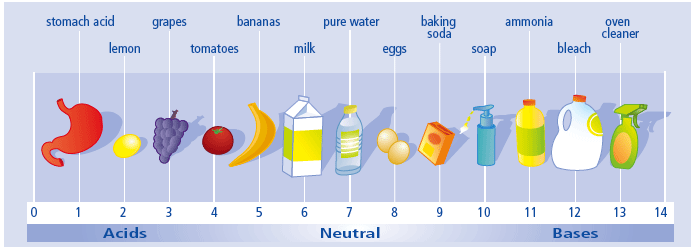
Acids and Bases
-
Water continuously dissociates to form H+ and OH- or even H30+ and OH- and re-associates back to H2O.




Try to memorize it!
Calculating pH
What is pH?
It is a measure of the hydrogen ion concentration of a solution.
Relationship between pH and pOH
pH + pOH = 14
[H+] + [OH-] = 10^-14
How to calculate pH?
-
To calculate the pH of an aqueous solution, you need to know the concentration of hydronium or hydrogen ion in moles per liter.
pH = - log [H3O+] or - log [H+]
Example:
Find the pH of a 0.0038 M HCl solution. The HCl is a strong acid and is 100% ionized in water. The hydronium ion concentration is 0.0038 M. Thus:
pH = - log (0.0038) = - ( - 2.42) = 2.42
Acid and Bases (continue)
Buffers are solutions that resist changes in pH by maintaining the concentrations of H+ ion.
-
If there is a lot of H+ ions, a buffer will absorb some of them to bring the pH back up.
-
If there is a little amount of H+ ions, a buffer will donate some H+ ions to bring the pH down.
How buffers work?
Carbonic acid and Bicarbonate buffer systems
Carbonic acid is a weak acid , which is produced when carbon dioxide reacts with water.

Carbonic acid releases H+ ion to form bicarbonate, which is a buffer.

This is what actually happen in your blood plasma to regulate your blood acidity.
Using tennis balls to demonstrate the blood buffer system
In AP Biology, you also need to know a bit about ocean acidification.

As more CO2 dissolves into the ocean, the more carbonic acid and bicarbonate ions are created. Since the ocean dissolves CO2 so quickly, bicarbonate ions (buffer) haven't been able to keep up. This happens because humans release a lot of CO2 to the environment. Excess carbonic acid reduces the ability of reef-building corals and corrodes the shells or skeletons of some organisms. This is extremely bad!
Ocean Chemistry
Ocean Acidification
Sources:
Reece, J. B., L. A. Urry, M. L. Cain, S. A. Waasserman, P. V. Minorsky, and R. B. Jackson. "Water and Life." In Campbell Biology, 93-104. 10th ed. San Francisco, CA: Pearson, 2011.