Chapter 4
Carbon and the Molecular Diversity of Life
Importance of Carbon
-
Carbon is so important and so difficult to understand at the same time. That's why we have organic chemistry, a branch of chemistry, just to learn about the properties, structure, compositions, reactions and formation of carbon-containing compounds.
I have two memes to describe what I just said:


However, you don't need to know organic chemistry to pass AP Biology, but you may need it to pass AP Chemistry.
-
Compounds that are organinc need to contain both carbon and hydrogen.
-
There are 6 major elements of life: CHNOPS (carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur).

Diversity of Carbon
-
Carbon is a versatile element because it can form up to 4 bonds.
-
It has 4 valence electrons.
-


-
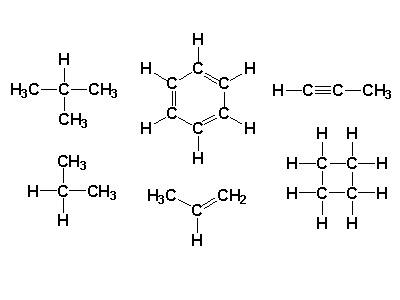
Those bonds can form single, double, and triple covalent bonds.

-
The structure can be a chain or a ring or a branch.

-
Carbon can also form large molecules.
-
There 4 major classes of macromolecules: carbohydrates, proteins, lipids, nucleic acids. (we will cover macromolecules in chapter 5)
-
-
Carbon forms isomers (đồng phân).
-
Molecule can have similar molecular formula but different arrangement.
-
This means that it has different properties or functions.
-
-
different structures -> different functions/properties

a) Structural isomers differ in the arrangement of the bonds.
b) Geometric isomers differ in the spatial arrangement around the double bonds.
c) Enantiomers are the reflection of each other and non-superimposable on one another.
-
Drug is an example of enantiomers.
-
Thalidomide:
-
This drug is used by pregnant women to reduce morning sickness. Since thalidomide has two possible enantiomers, it can cause birth defects.
-
"Good" enantiomer can change into "bad" in the body through chemical reactions.
-
-
More examples:
-

Functional groups
-
Properties of organic molecules are determined by functional groups.
-
There are 7 most common functional groups: hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, phosphate, methyl.


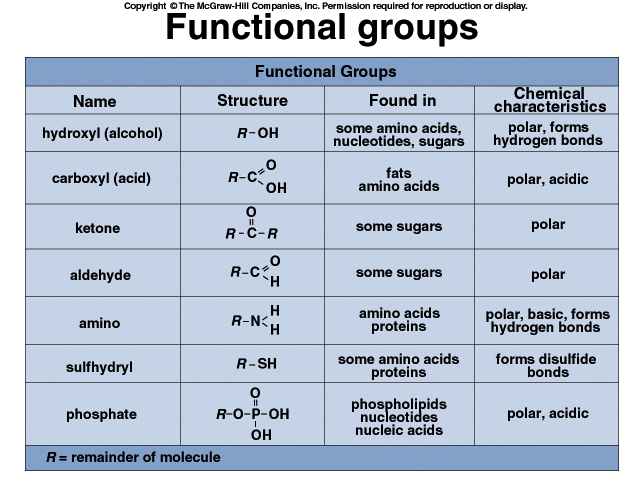
How to Understand and Memorize Functional Groups
Sources:
Reece, J. B., L. A. Urry, M. L. Cain, S. A. Waasserman, P. V. Minorsky, and R. B. Jackson. "Carbon and the Molecular Diversity of Life." In Campbell Biology, 105-114. 10th ed. San Francisco, CA: Pearson, 2011.